Abstract
Background: In patients with monoclonal gammopathy of undetermined significance (MGUS), the pre-malignant cells are antibody producing plasma cells. Stimulating humoral immunity mediated by plasma cells is an important factor in vaccine efficacy. Therefore, concerns have been raised about whether vaccine administration in patients with MGUS could trigger progression of the underlying pre-malignant clone, leading to an increase in the monoclonal-(M)-protein or even progression to full-blown malignancy. Immune stimulation has been found to be a risk factor for monoclonal gammopathies. However, immune stimulation inherent to vaccines and the subsequent antibody formation by plasma cells has, to our knowledge, not been studied in a systematic manner in MGUS. The current SARS-CoV-2 pandemic provide a unique opportunity to evaluate if SARS-CoV-2 vaccination has an impact on the risk of progression from MGUS to MM, or not.
To study this we performed a prospective nationwide study based on the Iceland Screens, Treats, or Prevents Multiple Myeloma (iStopMM) study.
Methods: The iStopMM study screened a total of 75,422 individuals for M protein and free light chains (FLC) between September 2016 and December 2020. The study diagnosed 3,358 individuals with MGUS and then randomized them to one of three arms. Individuals in arms 2 and 3 (N=2,037) are followed longitudinally with repeated serum protein electrophoresis (SPEP) and FLC and comprise the study population.
All SARS-CoV-2 vaccinations in Iceland are centrally registered, which gives complete nationwide information on vaccination coverage, type of vaccine used, and frequency of administration. By cross-linking the vaccine data to the iStopMM database, MGUS patients who had received at least one SARS-CoV-2 vaccination and had follow-up serum samples after vaccination were identified. As M protein levels change with age, linear mixed modelling was used to estimate changes in M protein concentration as a function of age before vaccination and after vaccination. The slope of the evolution of M protein level over age was modelled as a continuous linear effect and compared before and after each vaccination to determine the effects of SAR-CoV-2 vaccination on changes in M protein levels.
Results: A total of 1,814 SARS-CoV-2 vaccinated MGUS individuals were included in the study. The participants had 6,094 serum samples available with a median of 3 (range 1-21) per person (Table 1). All had at least one M protein measurement after receiving at least a single dose of vaccine. Median age at first vaccination was 71 (range 42-98) years and 53.9% were males. Median follow-up was 2.3 (range 0.5-5.5) years, and 98.9% of individuals had received at least two doses of SARS-CoV-2 vaccine. The most common vaccine (first dose) was BNT162b2 (Pfizer) 54.4%, followed by ChAdOx1 (Astra Zeneca) 38.0%, mrna-1273 (Moderna) 5.7% and AD26.COV2.S (Janssen) 1.9%.
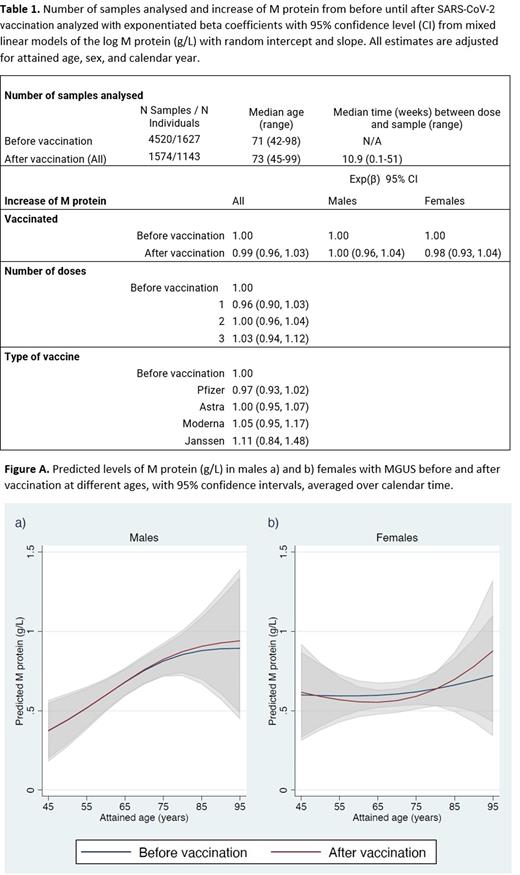
Comparing M protein levels pre- and post-vaccination showed a 1.9% increase in M protein concentration in males and 0.3% in females. However, there was no difference in the rate of M protein increase following vaccination compared to that observed before vaccination. This was true for both men and women (Figure A).
After adjusting for age, sex, and calendar year, no difference in M protein concentration was observed when comparing the increase before and after vaccination (Exponentiated beta coefficient (Exp(β)): 0.99; 95% confidence interval [CI], 0.96-1.03). The same was true when analyzed for each sex separately (Exp(β): 1.00; 95% CI, 0.96-1.04 for males and Exp(β) 0.98; 95% CI, 0.93-1.04 for females). No difference was observed between the different types of vaccines used. Similarly, neither second doses of vaccine (Exp(β): 1.00; 95% CI, 0.96-1.04) nor boosters (Exp(β): 1.03; 95% CI, 0.94-1.12) had an impact on the M protein concentration.
Conclusion: In this large, prospective, population-based, screening study including 1,814 individuals with MGUS who were observed before and after covid vaccinations, we found no evidence for MGUS progression after SARS-CoV-2 vaccination. The same was true when analyzed separately by sex, age, type of vaccine, and the number of doses given. Our findings indicate that SARS-CoV-2 vaccination is safe in individuals with MGUS and does not lead to progression irrespective of the number of vaccine doses administered and type of vaccine used.
Disclosures
Onundarson:Pall T. Onundarson MD: Patents & Royalties: patent for a new coagulation test, the Fiix prothrombin time. Hultcrantz:Curio Science LLC: Consultancy; Amgen, Daichii Sankyo, Cosette, GSK: Research Funding; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Intellisphere LLC: Consultancy; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Durie:Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Harding:The Binding Site: Current Employment, Membership on an entity's Board of Directors or advisory committees. Landgren:Leukemia & Lymphoma Society: Research Funding; Aptitude Health: Honoraria; MMRF: Honoraria; Rising Tide Foundation: Research Funding; Tow Foundation: Research Funding; Merck & Co., Inc.: Other: Independent Data Monitoring Committee (IDMC) member for clinical trials; Riney Foundation: Research Funding; NCI/NIH: Research Funding; Theradex: Other: Independent Data Monitoring Committee (IDMC) member for clinical trials; Janssen: Honoraria, Other: Independent Data Monitoring Committee (IDMC) member for clinical trials, Research Funding; Amgen: Honoraria, Research Funding; Pfizer Inc: Consultancy. Kristinsson:Celgene: Research Funding; Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.